Description
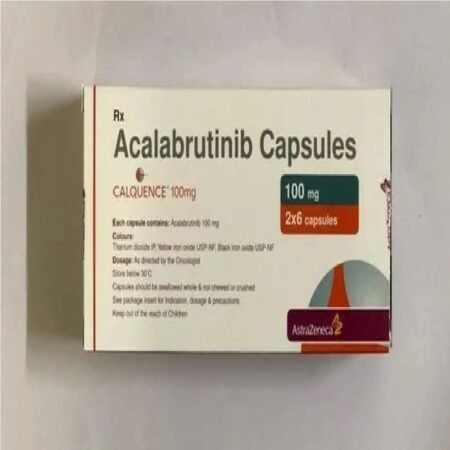
Acalabrutinib, sold under the brand name Calquence, is a medication used to treat various types of non-Hodgkin lymphoma, including mantle cell lymphoma (MCL) and chronic lymphocytic leukemia/small lymphocytic leukemia (CLL/SLL). It may be used both in relapsed as well as in treatment-naive settings. Common side effects include headaches, feeling tired, low red blood cells, low platelets, and low white blood cells. Acalabrutinib is a second generation Bruton’s tyrosine kinase inhibitor. Acalabrutinib blocks an enzyme called Bruton’s tyrosine kinase, which helps B cells to survive and grow. By blocking this enzyme, acalabrutinib is expected to slow down the build-up of cancerous B cells in CLL, thereby delaying progression of the cancer. Acalabrutinib was approved for medical use in the United States in 2017, and in the European Union in November 2020The most common adverse events were headache, diarrhea and weight gain. Despite the appearance of a greater occurrence of transient headaches, data suggest a preferred advantage of acalabrutinib over ibrutinib due to expected reduced adverse events of skin rash, severe diarrhea, and bleeding risk.
| Strength | Not specified |
| Pack Size | Not specified |
| Brand | Acabrunat |
| Packaging Type | Not specified |
| Composition | Not specified |
| Form | Not specified |
| Shelf Life | Not specified |
| Usages | Not specified |
| Country of Origin | Not specified |