Description
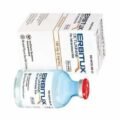
Cetuximab 200mg,100mg in combination with radiation therapy, is a monoclonal antibody indicated for the initial treatment of locally or regionally advanced head and neck cancer of a specific type (squamous cell carcinoma). Used alone, Erbitux is also approved to treat patients with head and neck cancers that have returned in the same location or spread to other parts of the body and for head and neck cancers that have progressed following platinum-based chemotherapy. Erbitux is also used on metastatic colorectal cancers that contain epidermal growth factor receptors. What are side effects of Erbitux? The most common side effects of Erbitux include: rash, itching, dry or cracked skin, nail changes, headache, diarrhea, nausea, vomiting, upset stomach, weight loss, weakness, and respiratory, skin, and mouth infections. Erbitux also can cause low blood magnesium, potassium, and calcium. Patients taking Erbitux should limit their exposure to the sun. Rare but serious side effects of Erbitux include: life-threatening allergic reactions and heart attacks, especially if the patient was also obtaining chemotherapy or radiotherapy. Recommended Dosage For Squamous Cell Carcinoma Of The Head And Neck (SCCHN) In Combination With Radiation Therapy Or Platinum-Based Therapy And Fluorouracil The recommended initial dose is 400 mg/m² administered one week prior to initiating a course of radiation therapy or on the first day of platinum-based therapy and fluorouracil as a 120-minute intravenous infusion. The recommended subsequent dosage (all other infusions) is 250 mg/m² weekly as a 60-minute infusion for the duration of radiation therapy (6–7 weeks) or until disease progression or unacceptable toxicity when administered in combination with platinum-based therapy and fluorouracil. Complete ERBITUX administration 1 hour prior to radiation therapy or platinum-based therapy with fluorouracil. Monotherapy The recommended initial dose is 400 mg/m² administered as a 120-minute intravenous infusion. The recommended subsequent dosage (all other infusions) is 250 mg/m² weekly as a 60-minute infusion until disease progression or unacceptable toxicity. Recommended Dosage For Colorectal Cancer (CRC) Determine EGFR-expression status using FDA-approved tests prior to initiating treatment. Also confirm the absence of a Ras mutation prior to initiation of treatment with ERBITUX. Information on FDA-approved tests for the detection of K-Ras mutations in patients with metastatic CRC /ucm301431.htm. The recommended initial dose, either as monotherapy or in combination with irinotecan or FOLFIRI (irinotecan, fluorouracil, leucovorin), is 400 mg/m² administered as a 120-minute intravenous infusion. The recommended subsequent dosage, either as monotherapy or in combination with irinotecan or FOLFIRI, is 250 mg/m² weekly as a 60-minute infusion until disease progression or unacceptable toxicity. Complete ERBITUX administration 1 hour prior to irinotecan or FOLFIRI.
| Strength | Not specified |
| Pack Size | Not specified |
| Brand | Not specified |
| Packaging Type | Box |
| Composition | Not specified |
| Form | Not specified |
| Shelf Life | Not specified |
| Usages | Not specified |
| Country of Origin | Not specified |